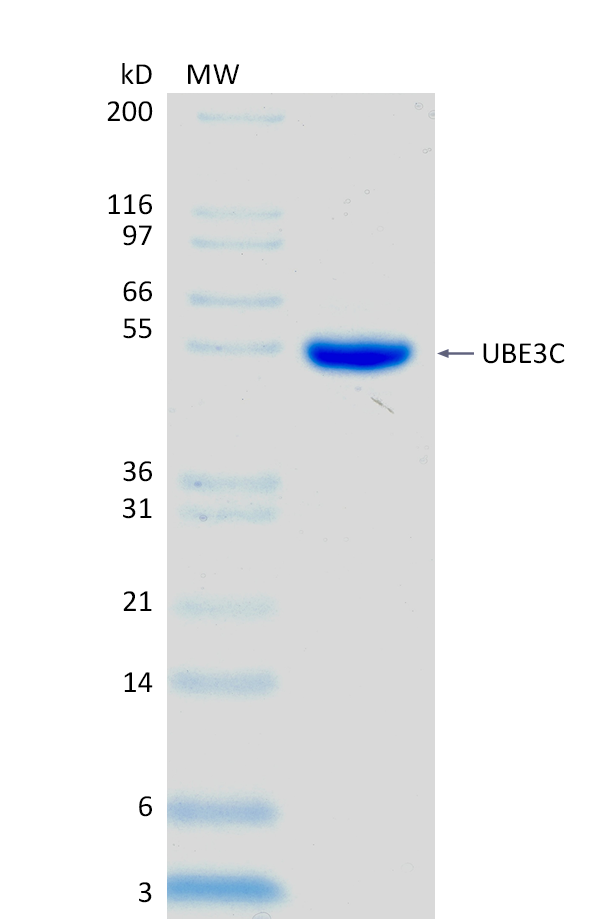
 2 μg UBE3C run on 4-12% SDS-PAGE gel under reducing conditions, then visualized with Colloidal Coomassie Blue Stain.
2 μg UBE3C run on 4-12% SDS-PAGE gel under reducing conditions, then visualized with Colloidal Coomassie Blue Stain.For Research Use Only (RUO)
Chu, B. W., et al., (2013) J Biol Chem 288:34575-87. PMID 24158444
Singh, S., and J. Sivaraman (2020) Biochem J 477:905-23. PMID 32039437
Hang, C., et al., (2021) Cancer Cell Int 21:25. PMID 33407510
Cui, B., et al., (2024) J Virol 98:e0133524. PMID 39212385